Alkali Metals Densities . Are soft (they can be cut with a knife) have relatively low. Alkali metals have one electron in their outer shell, which is loosely bound. The heavier chalcogens (group 16) to produce metal chalcogenides; These are all light elements. the alkali metals react with halogens (group 17) to form ionic halides; — group 1 alkali metals. Lithium, sodium and potassium are all less dense than water, and so float on water. The group 1 elements, also known as the alkali metals, all react vigorously with water to. — the alkali metals: And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. — the alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals.
from demonstrations.wolfram.com
And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. The heavier chalcogens (group 16) to produce metal chalcogenides; Are soft (they can be cut with a knife) have relatively low. Alkali metals have one electron in their outer shell, which is loosely bound. — the alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. The group 1 elements, also known as the alkali metals, all react vigorously with water to. the alkali metals react with halogens (group 17) to form ionic halides; Lithium, sodium and potassium are all less dense than water, and so float on water. — the alkali metals: — group 1 alkali metals.
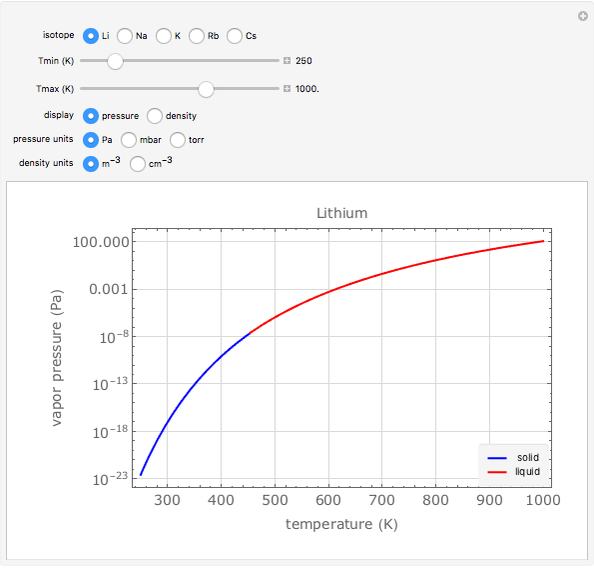
Vapor Pressure and Density of Alkali Metals Wolfram Demonstrations
Alkali Metals Densities The group 1 elements, also known as the alkali metals, all react vigorously with water to. Are soft (they can be cut with a knife) have relatively low. These are all light elements. The heavier chalcogens (group 16) to produce metal chalcogenides; — the alkali metals: And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. Lithium, sodium and potassium are all less dense than water, and so float on water. — the alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. the alkali metals react with halogens (group 17) to form ionic halides; The group 1 elements, also known as the alkali metals, all react vigorously with water to. Alkali metals have one electron in their outer shell, which is loosely bound. — group 1 alkali metals.
From online-learning-college.com
Group 1 alkali metals Properties of alkali metals Reactions Alkali Metals Densities Are soft (they can be cut with a knife) have relatively low. — the alkali metals: — group 1 alkali metals. The group 1 elements, also known as the alkali metals, all react vigorously with water to. Alkali metals have one electron in their outer shell, which is loosely bound. the alkali metals react with halogens (group. Alkali Metals Densities.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Alkali Metals Densities And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. Are soft (they can be cut with a knife) have relatively low. — the alkali metals: These are all light elements. — group 1 alkali metals. Alkali metals have one electron in their outer shell, which is loosely bound. The heavier chalcogens (group. Alkali Metals Densities.
From present5.com
THE ALKALI METALS Where are the alkali metals? Alkali Metals Densities the alkali metals react with halogens (group 17) to form ionic halides; — group 1 alkali metals. These are all light elements. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. The group 1 elements, also known as the alkali metals, all react vigorously with water to. Are soft (they can be. Alkali Metals Densities.
From www.britannica.com
alkali metal Definition, Properties, & Facts Britannica Alkali Metals Densities — the alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. — the alkali metals: Are soft (they can be cut with a knife) have relatively low. — group 1 alkali metals. the alkali metals react with halogens (group 17) to form ionic halides;. Alkali Metals Densities.
From slideplayer.com
Groups I & II, the Alkali Metals and the Alkaline Earth metals ppt Alkali Metals Densities And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. — the alkali metals: Alkali metals have one electron in their outer shell, which is loosely bound. — group 1 alkali metals. The group 1 elements, also known as the alkali metals, all react vigorously with water to. These are all light elements.. Alkali Metals Densities.
From www.slideserve.com
PPT Where are the alkali metals? PowerPoint Presentation ID5525447 Alkali Metals Densities Alkali metals have one electron in their outer shell, which is loosely bound. the alkali metals react with halogens (group 17) to form ionic halides; The group 1 elements, also known as the alkali metals, all react vigorously with water to. These are all light elements. — group 1 alkali metals. The heavier chalcogens (group 16) to produce. Alkali Metals Densities.
From www.slideshare.net
The periodic table and identification of ions Alkali Metals Densities The heavier chalcogens (group 16) to produce metal chalcogenides; — the alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. — group 1 alkali metals. Are soft (they can be cut with a knife) have relatively low. The group 1 elements, also known as the alkali. Alkali Metals Densities.
From overallscience.com
Trends in atomic and physical properties of alkali metals Overall Science Alkali Metals Densities Lithium, sodium and potassium are all less dense than water, and so float on water. the alkali metals react with halogens (group 17) to form ionic halides; Are soft (they can be cut with a knife) have relatively low. The group 1 elements, also known as the alkali metals, all react vigorously with water to. And oxygen to form. Alkali Metals Densities.
From www.vedantu.com
Alkali Metals Chemical Elements, Properties Alkali Metals Periodic Alkali Metals Densities Are soft (they can be cut with a knife) have relatively low. — group 1 alkali metals. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. The heavier chalcogens (group 16) to produce metal chalcogenides; The group 1 elements, also known as the alkali metals, all react vigorously with water to. —. Alkali Metals Densities.
From xlskoor.blogspot.com
Alkali Metals Chemistry Alkali Metals Densities — group 1 alkali metals. These are all light elements. The group 1 elements, also known as the alkali metals, all react vigorously with water to. The heavier chalcogens (group 16) to produce metal chalcogenides; — the alkali metals: Lithium, sodium and potassium are all less dense than water, and so float on water. Alkali metals have one. Alkali Metals Densities.
From www.slideserve.com
PPT Melting Points of Alkali Metals PowerPoint Presentation, free Alkali Metals Densities Are soft (they can be cut with a knife) have relatively low. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. Lithium, sodium and potassium are all less dense than water, and so float on water. These are all light elements. Alkali metals have one electron in their outer shell, which is loosely bound.. Alkali Metals Densities.
From www.researchgate.net
the calculated densities for alkali metals compared with the Alkali Metals Densities These are all light elements. Alkali metals have one electron in their outer shell, which is loosely bound. — group 1 alkali metals. Are soft (they can be cut with a knife) have relatively low. — the alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals.. Alkali Metals Densities.
From studylib.net
Alkali metals Alkali Metals Densities The group 1 elements, also known as the alkali metals, all react vigorously with water to. The heavier chalcogens (group 16) to produce metal chalcogenides; And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. — the alkali metals exhibit many of the physical properties common to metals, although their densities are lower than. Alkali Metals Densities.
From www.slideserve.com
PPT Melting Points of Alkali Metals PowerPoint Presentation, free Alkali Metals Densities The heavier chalcogens (group 16) to produce metal chalcogenides; Lithium, sodium and potassium are all less dense than water, and so float on water. — the alkali metals: — group 1 alkali metals. Are soft (they can be cut with a knife) have relatively low. — the alkali metals exhibit many of the physical properties common to. Alkali Metals Densities.
From www.slideserve.com
PPT Group 1 The alkali metals PowerPoint Presentation ID5525387 Alkali Metals Densities These are all light elements. — group 1 alkali metals. Are soft (they can be cut with a knife) have relatively low. The group 1 elements, also known as the alkali metals, all react vigorously with water to. The heavier chalcogens (group 16) to produce metal chalcogenides; Lithium, sodium and potassium are all less dense than water, and so. Alkali Metals Densities.
From askfilo.com
The increasing order of the density of alkali metals Filo Alkali Metals Densities the alkali metals react with halogens (group 17) to form ionic halides; The heavier chalcogens (group 16) to produce metal chalcogenides; The group 1 elements, also known as the alkali metals, all react vigorously with water to. Alkali metals have one electron in their outer shell, which is loosely bound. Lithium, sodium and potassium are all less dense than. Alkali Metals Densities.
From agmetals.com
Density of Precious Metals Alkali Metals Densities Lithium, sodium and potassium are all less dense than water, and so float on water. Alkali metals have one electron in their outer shell, which is loosely bound. — group 1 alkali metals. The heavier chalcogens (group 16) to produce metal chalcogenides; These are all light elements. — the alkali metals exhibit many of the physical properties common. Alkali Metals Densities.
From sciencestruck.com
Properties of Alkali Metals Science Struck Alkali Metals Densities — group 1 alkali metals. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. Lithium, sodium and potassium are all less dense than water, and so float on water. — the alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals.. Alkali Metals Densities.